Hope for the Huntington’s Disease Community
November 2025
By Alexa Dreyfus
Edited by Allen Zhao
A new breakthrough treatment has been developed that aims to slow the progression of Huntington’s disease. Last month, a company based in Amsterdam, uniQure, released a data brief on its new AMT-130 treatment. AMT-130 is based on a mechanism that halts the part of gene production that is responsible for producing the harmful huntingtin protein. It accomplishes this targeting through attacking the mRNA, something our bodies use that carries genetic information, by preventing the formation of DNA without alteration and avoiding risks of gene editing. It is vital to avoid these risks as gene editing can trigger negative effects such as changing DNA sequencing, causing cancer, or causing immune reactions. This treatment, AMT-130, was reported to slow the progression of Huntington’s to a minuscule pace.
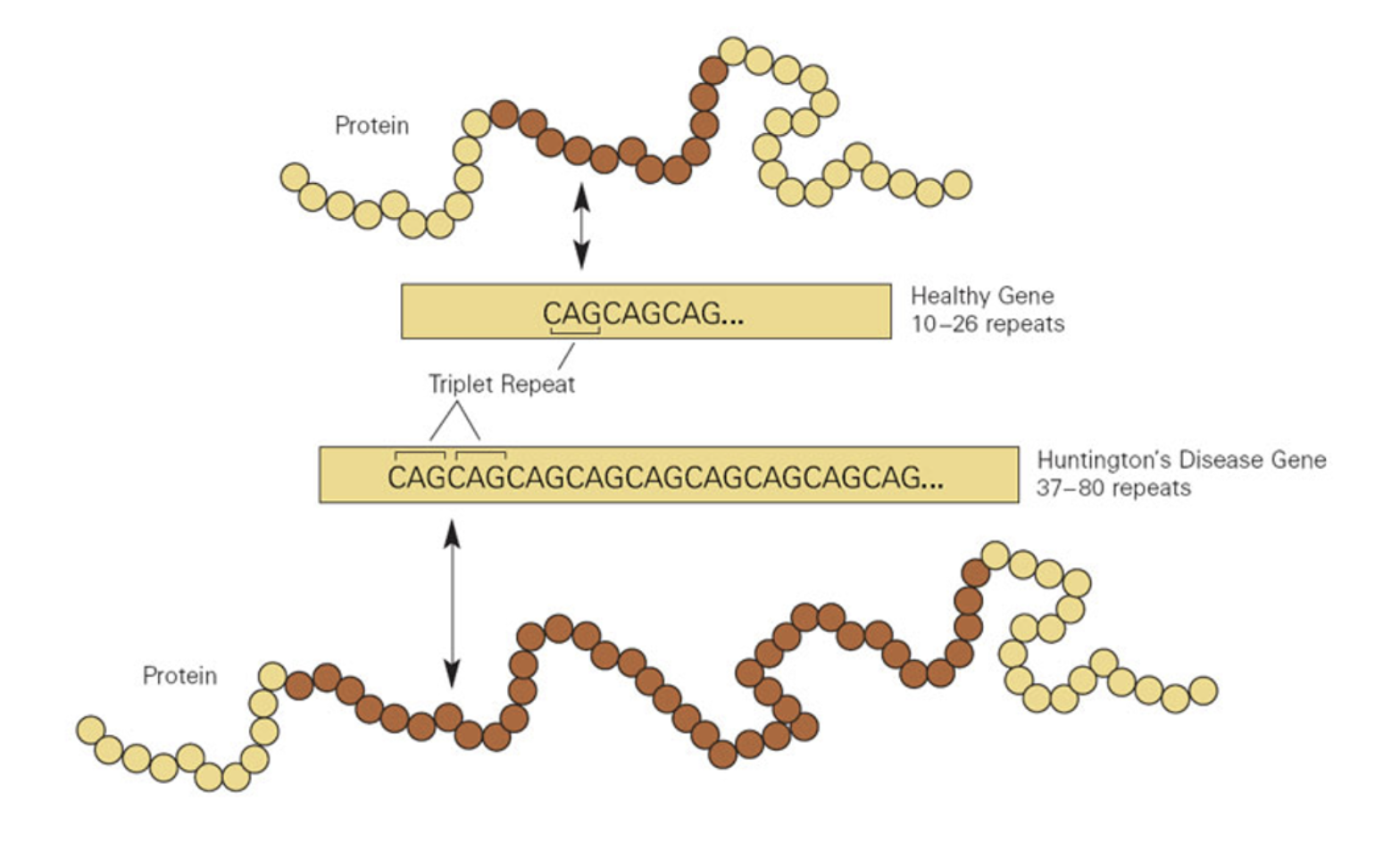
In the United States alone, there are approximately 41,000 individuals who suffer from Huntington’s. Huntington’s is a fatal genetic disease, passed on from generation to generation. This debilitating disease comes along with symptoms such as movement and cognitive impairments, seeping in and affecting every aspect of everyday life. Movement symptoms may include jerking movements, muscle contracture, unusual eye movements, trouble walking, and trouble with speech or swallowing. Further, cognitive impairments may include difficulty organizing tasks, lack of flexibility, lack of impulse control, lack of awareness, slowness in processing thoughts, and trouble learning new information. The disease symptoms typically develop as individuals are in their 30s and 40s, with different rates of progression.Figure 1. The image shows the genetic mutation responsible for Huntington's disease, the excessive repetitions of the CAG nucleotides.
Figure 1. The image shows the genetic mutation responsible for Huntington's disease, the excessive repetitions of the CAG nucleotides.
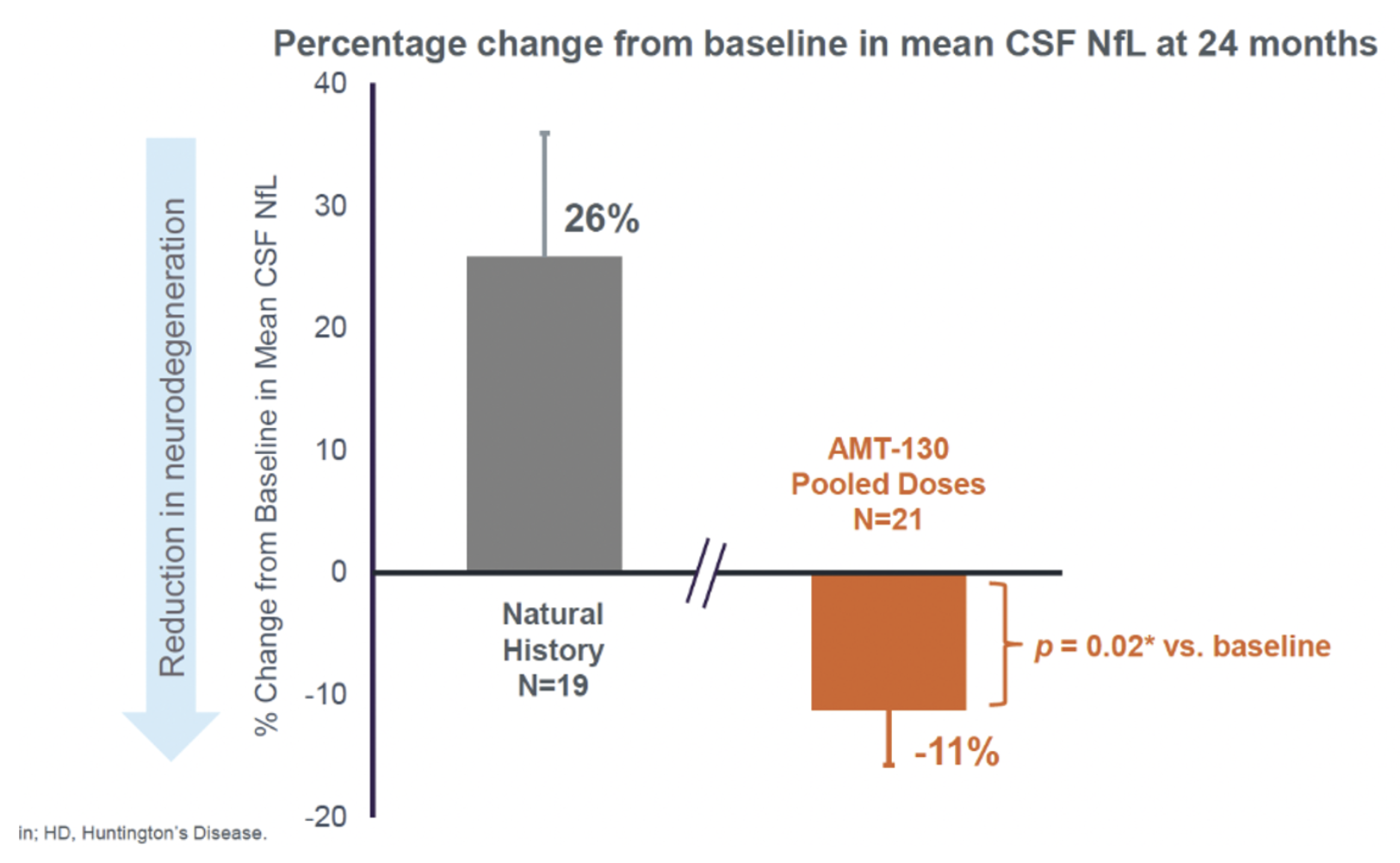
Due to the severe nature of this disease, uniQure has focused efforts on creating a clinical trial to search for results that can provide meaningful results to patients. The clinical trial had 29 participants, with some receiving a low dose and some receiving a high dose through brain surgery. The participants who received the higher dose of treatment in the trial had a 75 percent at 36 months and slower disease progression compared to the control group that did not receive the treatment. This was measured by the total functional capacity of the patients, treated and untreated, which yielded favorable results.Figure 2. This graph is from a UniQure study update detailing Cerebral Spinal Fluid neurofilament light chains(Nfl). Nfls are biomarkers for Huntington's and are significantly down compared to control.
Figure 2. This graph is from a UniQure study update detailing Cerebral Spinal Fluid neurofilament light chains(Nfl). Nfls are biomarkers for Huntington's and are significantly down compared to control.
The treatment was developed to be a single dose that lasts for a lifetime, without requiring a daily medication. While this one-time treatment has major benefits, it is also invasive to the patients. Although the treatment has not yet been approved by the FDA, an accelerated approval for the drug is being sought after. This development is an incredible leap in the right direction towards slowing the disease progression of Huntington’s disease, but still highlights the required progress regarding providing the treatment to patients. Nevertheless, AMT-130 has the potential to transform treatments for Huntington’s disease. This breakthrough is evidence that this treatment approach can be applied to a range of neurological disorders, providing proof regarding one-time gene therapies.
The response from the community has been immensely positive, as not only those that are diagnosed with Huntington’s disease, but those that have loved ones affected or are at risk of testing positive for the gene have experienced a new beacon of hope for the future. Previously, receiving a Huntington’s disease diagnosis was incredibly dismal news to be hearing from a physician. Thus, the development of treatment that has the possibility to improve the lives of those affected by the disease by slowing progression is an incredible step in the right direction for treating neurological diseases. While this is a promising development, it is crucial to remain cautious as the results are not yet peer-reviewed and there is minimal information regarding side effects of the invasive treatment.
References
Devlin, H. (2025, September 24). Huntington's disease treated successfully for first time in UK gene therapy trial. The Guardian. Retrieved November 2, 2025, from https://www.theguardian.com/science/2025/sep/24/huntingtons-disease-treated-successfully-for-first-time-in-gene-therapy-trial
Gaither, K. (2025, October 8). Breakthrough in Huntington's disease treatment shows unprecedented results for patients. The University of Alabama at Birmingham. Retrieved November 2, 2025, from https://www.uab.edu/news/research-innovation/breakthrough-in-huntingtons-disease-treatment-shows-unprecedented-results-for-patients
Huntington's disease - Symptoms and causes. (2024, April 25). Mayo Clinic. Retrieved November 2, 2025, from https://www.mayoclinic.org/diseases-conditions/huntingtons-disease/symptoms-causes/syc-20356117
National Institute of Standards and Technology. “Huntington’ s Disease.” NIST, 12 Apr. 2011, Image gallery, https://www.nist.gov/image/huntingtons-disease
Simmons, L., & Evans, K. (2025, September 25). Huntington’ s Disease Successfully Treated For First Time – Gene Therapy Slows Progression By 75 Percent. IFLScience. Retrieved November 2, 2025, from https://www.iflscience.com/first-ever-successful-huntingtons-disease-treatment-slows-progression-by-75-percent-80942
uniQure N.V . “uniQure Announces Positive Topline Results from Pivotal Phase I/II Study of AMT-130 in Patients with Huntington’s Disease.” uniQure: Investors & Media, 24 Sept. 2025. Web. https://uniqure.gcs-web.com/news-releases/news-release-details/uniqure-announces-positive-topline-results-pivotal-phase-iii